SURAMIN
for
AUTISM
Cell Danger Response
Real Science. Real Change.
For the first time, a treatment is showing clear and measurable improvements in core autism symptoms, speech, connection, and awareness through the use of Suramin.
Families around the world are seeing progress they never thought possible. Backed by peer-reviewed research and growing global interest, Suramin is bringing new hope to the autism community.
Speech Before SURAMIN
Speech After SURAMIN
Interview With Dr Robert Naviaux
Autism Spectrum Disorder (ASD)
Drug: Placebo
Drug: Suramin
Suramin for the Treatment of Autism Trial: KZ101 in a Male Pediatric Population With Autism Spectrum Disorder (ASD) (STAT-2A)
Participate in a Clinical Trial
Suramin has been found to correct the symptoms, metabolism, and brain synaptic abnormalities in two classical genetic and environmental mouse models of autism. A preliminary clinical trial (SAT-1) examined the safety and activity of a single low-dose of suramin in children with ASD and concluded suramin showed promise as a novel approach to treatment of ASD. The current study, STAT-2A, will be a randomized, double-blind, crossover, 30-week study to evaluate the preliminary proof of concept, safety, and PK of suramin sodium (KZ101) with repeat dosing by IV infusion in males 5–14 years of age who have been diagnosed with ASD. The study will be conducted at approximately 3 sites contributing approximately 15 subjects per site. Total enrollment of approximately 45 subjects is planned to achieve approximately 36 participants completing the study.Full description
After up to a 4-week screening period, participants will undergo 8 weeks of active or placebo treatment (Period 1), followed by an 8-week washout period, and then cross over to 8 weeks of placebo or active treatment (Period 2). Patients will be followed for 2 weeks after completion of Period 2. Two dosing groups are designated as Group A, who are randomly assigned to active treatment with KZ101 in Period 1 and saline in Period 2, and Group B, who are randomly assigned to saline infusion in Period 1 and active treatment with KZ101 in Period 2. Dosing in both periods will consist of 2 IV infusions of either saline (placebo) or KZ101 (active treatment), given 4 weeks apart.
© Suramin for Autism. All rights reserved.

Autism Research – SURAMIN
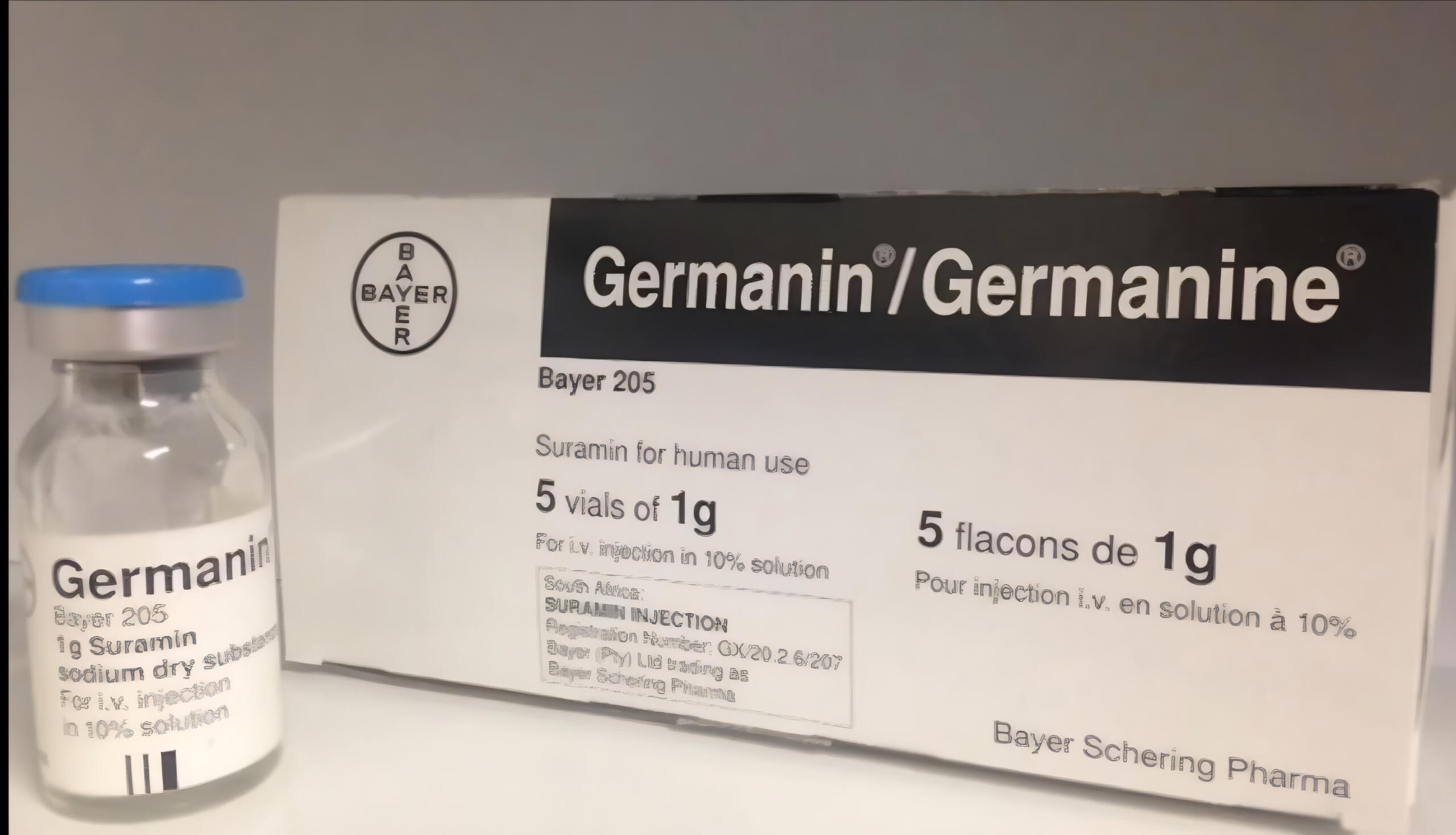
Suramin for Autism: A Breakthrough That’s Hard to IgnoreFor decades, autism research has been stuck in a frustrating loop with endless studies and support programs, but little to offer in terms of real treatment for the root causes. Families were told to manage, cope, adjust. Progress, if any, was slow and uncertain.But all of that began to change with one unexpected discovery: an old drug called Suramin.---A 100-Year-Old Drug, a Brand-New PurposeOriginally developed to treat African sleeping sickness in the early 1900s, Suramin had largely faded from modern medicine until Dr. Robert Naviaux at UC San Diego made a remarkable observation.His research revealed that autism may be tied to a chronic biological state called the Cell Danger Response a condition where the body gets stuck in “survival mode” long after the original stress is gone. When this switch stays on, it can interfere with development, learning, speech, and social interaction.Suramin, it turns out, can turn that signal off.---What Happened When Children Took SuraminIn 2017, Dr. Naviaux and his team ran a small but groundbreaking study. Five boys with moderate-to-severe autism received a single, carefully dosed infusion of Suramin. What happened next shocked everyone even the researchers.Within days, families reported life-changing changes:A child who had never spoken said his first full sentenceA boy who avoided touch hugged his mom for the first timeKids started singing, playing, making jokes, asking questionsStandardized autism tests confirmed what parents were seeing. Language improved. Social behaviors expanded. Repetitive patterns decreased.These weren’t just subtle shifts they were significant, and they happened fast.---Why It Works And Why It’s DifferentWhat makes Suramin so different from other “autism treatments” is that it doesn’t aim to cover up symptoms. It goes deeper, targeting the metabolism of cells and resetting key signals that affect brain development and inflammation.This is not a mystery drug. It’s not hype. The science behind it is solid, measurable, and carefully documented.And that’s what makes it so hopeful not just for autism, but potentially for other conditions where the body gets stuck in survival mode long after the original injury, infection, or trauma.---The single low dose lasted about six weeks. After that, the children gradually returned to baseline. But parents weren’t devastated they were energized. Because now they had proof: progress was possible. Their child’s potential had just been unlocked even if just for a moment.That moment changed everything.---Where Things Stand NowLarger, more detailed studies are already happening. A Phase II trial with dozens of children is expected to give clearer guidance on dosage, safety, and long-term benefits.Suramin is not yet approved for autism, and it must be used under medical supervision. But the doors it has opened are already reshaping how we think about treatment, recovery, and what’s truly possible.---Yes, there’s more work ahead. Yes, families should be cautious. But this is the first time we’ve seen something that truly addresses the root biology of autism.This is hope with evidence behind it.---A New Future for Autism TreatmentThe story of Suramin is still being written but it already offers something rare: proof of possibility.If a single dose of an old forgotten medicine can unlock language, connection, and joy in just days… imagine what lies ahead with ongoing care, more research, and broader access.The question is no longer “Can autism improve?”
It’s “How far can we go?”---Want to stay in the loop?
Sign up for updates, follow the trials, or reach out directly to learn more. A brighter path is finally here and it’s just beginning.
About Suramin for Autism
Clinical Trials: The Scientific Foundation Behind Suramin for AutismSuramin is not a new compound but its role in autism treatment represents one of the most promising scientific breakthroughs in decades. Multiple clinical trials have now demonstrated that Suramin can significantly improve core symptoms of Autism Spectrum Disorder (ASD) by targeting the metabolic pathways underlying the condition.This page outlines the major trials to date, with links to peer-reviewed publications, trial registries, and research summaries.
---🔬 Phase I/II Trial UC San Diego (SAT-1 Study)Principal Investigator: Dr. Robert K. NaviauxInstitution: University of California, San Diego (UCSD)Year: 2017Participants: 10 boys with moderate to severe ASD, ages 5–14Method: Randomized, double-blind, placebo-controlledIntervention: Single intravenous dose of Suramin (2 mg/kg)Outcomes:Statistically significant improvement in:
Language and communicationSocial interactionRepetitive and restrictive behaviorsGains were observed within days and lasted approximately 5–6 weeksChanges confirmed using validated tools: ADOS-2, ABC Core, CGISafety:No serious adverse eventsOne child experienced a mild, self-limiting rash📄 Published Study: Annals of Clinical and Translational Neurology (2017)
📄 Full Protocol: SAT-1 Clinical Trial Registry
---🧪 Phase II Trial — PaxMedica (PAX-101 Study)Sponsor: PaxMedica Inc.Location: United StatesYear: 2023–2024Participants: 52 children with ASDMethod: Randomized, placebo-controlledIntervention: Monthly IV infusions of Suramin (PAX-101) over 14 weeksKey Findings:48% average improvement in core autism symptoms in the Suramin group (vs. 31% in placebo)A larger proportion of the Suramin group saw >70% improvementStatistically significant improvement in social and behavioral domainsNo serious adverse events reported📄 Trial Summary: PaxMedica News Release
📄 Trial ID: ClinicalTrials.gov NCT04695181
---Ongoing International TrialsSuramin for Autism – South Africa (2024–2025)Design: Multicenter, randomized, placebo-controlledParticipants: 52 children with ASDDosing Arms: 1mg/kg and 2mg/kg intravenous SuraminPrimary Outcomes: Autism symptom severity, safety, and metabolic biomarkersThis study is aimed at confirming efficacy across broader populations and establishing standardized dosing protocols.📄 Trial ID: ClinicalTrials.gov NCT04595513
---Mechanism of ActionAll trials to date are based on a common biological model: the Cell Danger Response (CDR). According to Dr. Naviaux’s research, autism is linked to a chronic activation of the CDR, which blocks communication between cells and impairs development.Suramin blocks purinergic signaling, which is the core driver of the CDR. This allows cells to resume normal growth, immune regulation, and communication.📄 Mechanism Summary: Naviaux RK. “Metabolic features of the cell danger response” (Mitochondrion, 2014)
---What This MeansSuramin is currently the only drug that has demonstrated improvements in core autism symptoms (not just behavior or comorbidities) in controlled human trials.These studies provide strong justification for expanded access programs, future FDA submissions, and international research collaboration.
---For Researchers & Medical Professionals:We welcome collaboration with institutions, clinics, and ethics boards seeking to expand or replicate these trials.Contact us to receive trial protocols, white papers, or arrange direct consultation with researchers.SURAMIN COST BREAKDOWN – PLEASE READWe’ve secured a trusted Suramin source in South Africa, and are now launching real-world dosing and parent-led trials in the U.S.📍 Trial StatesCaliforniaFloridaMichiganEach trial includes 3 doses over 6 months, with 15 participants per state. The starter dose(×2) is more available
Priority goes to non-verbal individuals.Contribution CostThis effort is fully community-funded your contribution covers sourcing, import, sterile prep, clinical support, and dosing guidance.Self-Administered (Shipped): $810 totalCalifornia Trial (Local): $720 totalFlorida & Michigan Trials: $810 totalPay $729 upfrontRemainder ($120–$229) paid at the clinicYou're not just receiving Suramin, you’re helping build a path forward for families everywhere.Disclaimer: Suramin is not yet approved by the FDA or EMA for the treatment of autism.
Contact
Welcome!
We’re glad you’re here. Just a few quick questions to get you started this will only take a moment and helps us serve you better.
Join our WhatsApp community for new updates.
Thank you
Thank you for filling out the form!
We’ve received your information and will be in touch with you shortly.
For faster updates and exclusive info, feel free to join our WhatsApp community here